Mechanism of a molecular valve in the
halorhodopsin
chloride pump.
Andreea D. Gruia, Ana-Nicoleta Bondar, Jeremy C. Smith and
Stefan
Fischer*
Stucture 13, p. 617-627
(2005).
Full
paper (PDF)
Abstract
Halorhodopsin is an archaeal rhodopsin that uses light energy to pump
chloride
ions across the cellular plasma membrane during a photocycle triggered
by retinal photoisomerization.
The mechanism by which chloride is transferred across the retinal
chromophore
after photoisomerization (the primary transfer step) is explored
by computing all possible Minimum-Energy Pathways (MEP) for
this
process, using the
Conjugate Peak Refinement (CPR)
method. This reveals that one pathway has an activation
barrier (~9kcal/mol) significantly lower than the others.
Along this path, the chloride anion, driven by an interaction with
the protonated Schiff base, transiently opens a passage between Ser115
and retinal, which is not present in the ground state structure. To
allow
this opening, flexible deformation of the protein more than 10Angstroem
around retinal is necessary, mostly involving helix C.
This flexible deformation raises the chloride translocation
barrier when retinal is in the all-trans ground-state.
Unlike
macroscopic valve designs, chloride back-flow between photocycle is
slowed
down by the protein deformation, which serves as a valve spring.
This spring is tuned to allow differential ion flows in the pumping
versus
resting states that match the physiological times scales of the cell,
thus
creating a "kinetic" valve.
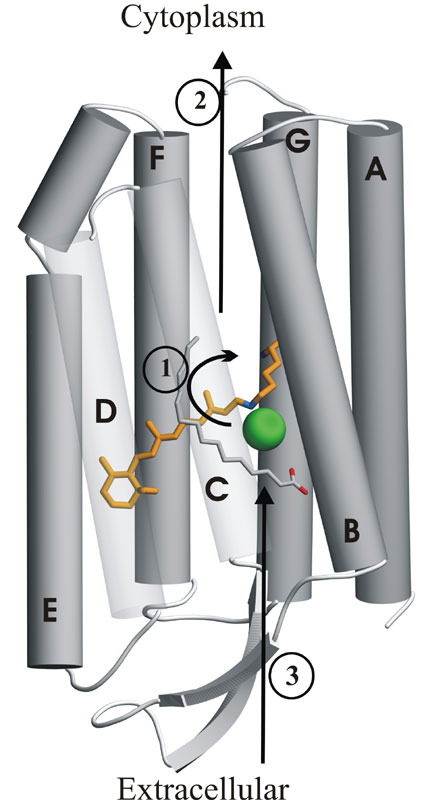
| The all-trans retinal chromophore
(orange) is
covalently attached via a protonated Schiff base (blue) to Lys242 on
helix
G.
Absorption of a photon trigers isomerization of the C13-C14
double bond
of retinal into the 13-cis conformation. This
leads
to a photocycle during which one chloride ion (green) is sequentially
transfered
from the extracellular to the cytoplasmic side of the membrane.
The arrows shows the chloride transfer in the order in which
they occur.
Step
1 is the 'primary' transfer step past the 13-cis retinal.
|
Chloride routes along the four different Minimum Energy
Pathways found
by the Conjugate Peak Refinement (CPR)
method.
For each route, the chain of small spheres shows the position
of the
chloride, the bigger sphere indicating the position at the transition
state.
|
|
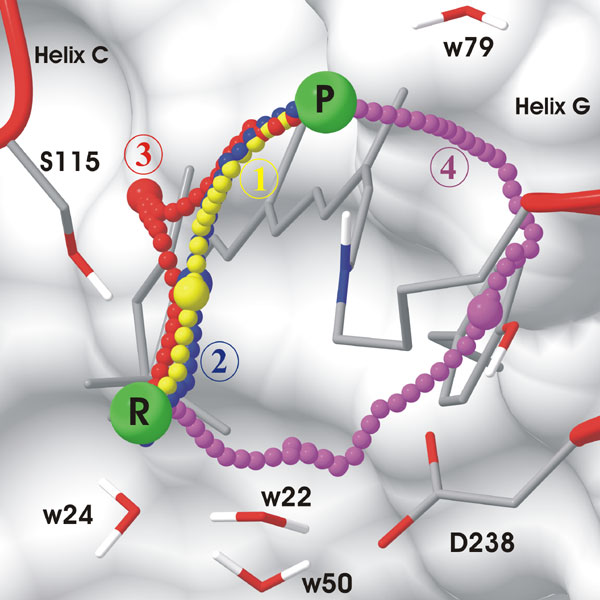 |
Prefered path of the primary chloride transfer
during
pumping (retinal is 13-cis):
Path 1 has the lowest energy barrier (~9.2 kcal/mol).
To be seen: Chloride passes on the Ser115 side of retinal, in front
of the Ser115 sidechain. Chloride transiently enlarges a lumen by
pushing
away helix C and the palmitate. H-bonds with water w24 and
Ser115
are maintained until after the transition state is passed and the lumen
starts reclosing. They are replaced by a salt-bridge with the
Schiff
base and water w79. Path 2 is very similar to path 1.
Download the movie , 1Mb
Chloride leaking in the resting state between
photocycles
(retinal in all-trans):
The energy barrier is 28kcal/mol, resulting in a very low rate
of leaking, on the timescale of hours. This prevents chloride
backflow while the protein waits to be actived by the next photon.
The route of the chloride is similar to that of path 1.
Download the movie , 1Mb
Energetically unfavorable paths for primary
transfer
(retinal is 13-cis):
Path 3 has an energy barrier of 17.2 kcal/mol, thus this
path is unlikely.
To be seen: The chloride follows a route in the back of the sidechain
of Ser115.
Download the movie , 1.6Mb
Path 4 has an energy barrier of 41.3 kcal/mol, thus
this
path is excluded. There is no lower MEP with a chloride route on
the Asp238 side of retinal. The electrostatic repulsion between
Asp238
and the chloride anion is responsible for the high barrier.
To be seen: Chloride goes through the water cluster, Asp238 then
bends away from the approaching anion and the chloride squeezes between
retinal and Tyr210.
Download the movie , 1.6Mb
Conclusions
Collective motion of helices C and G allow the transient enlargement
of a lumen near the Schiff base, on the Ser115 side of retinal,
requiring
flexible deformation of the protein up to 10 Angstroem around the
Schiff
base. This is facilitated by a built-in "breaking-point" in helix C,
due
to Pro117, located right next to the Schiff base. For a
description
of the kinetic valve mechanism, see the paper.
Go to Home of S. Fischer

