Proteins, vol. 59, p. 534-544 (2005).
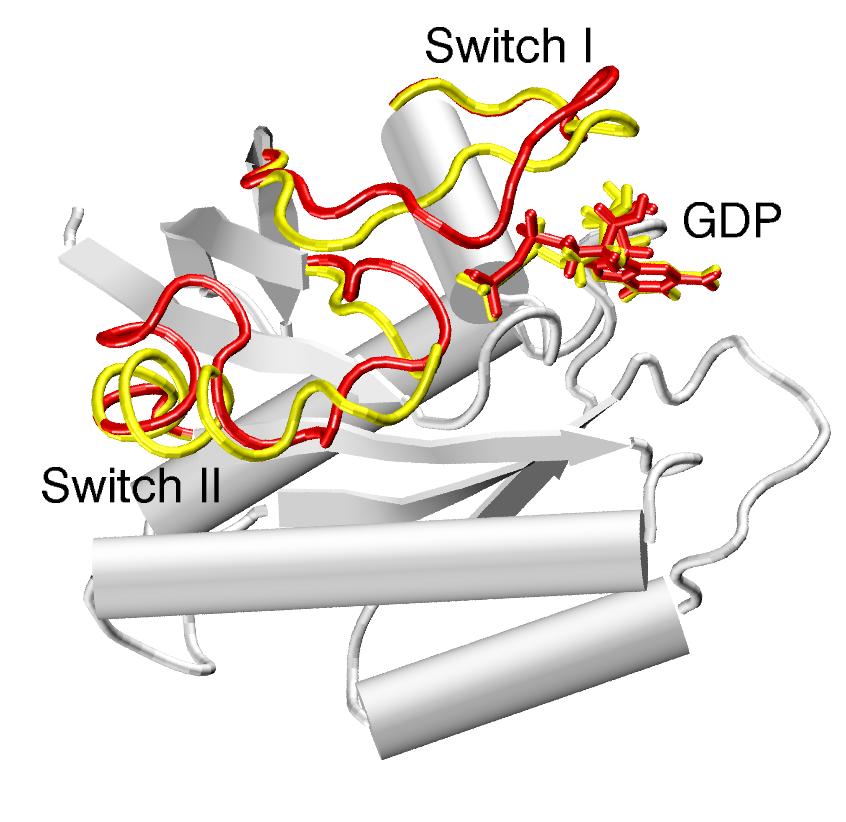
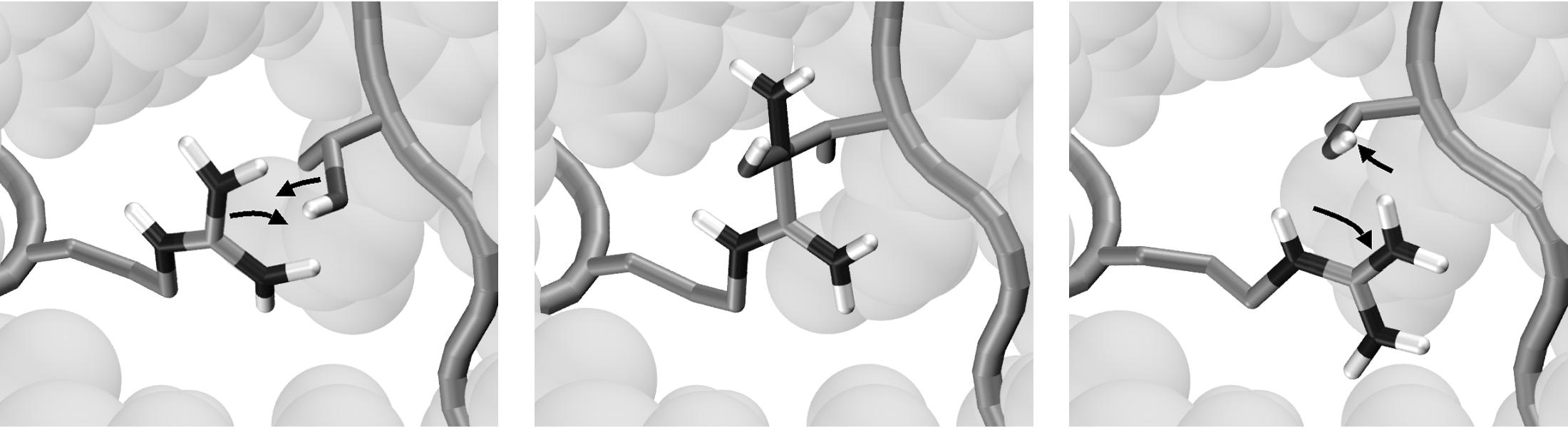
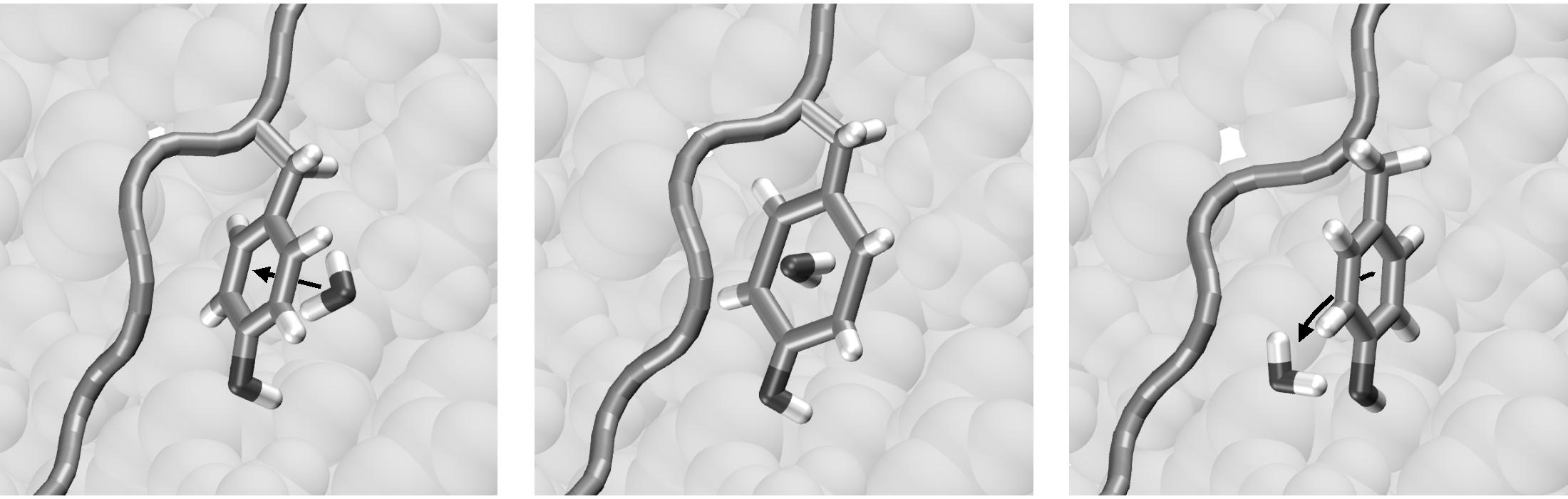
| The two end-states: Ras p21 is a crucial signaling-protein involved in regulating cell reproduction. Upon hydrolysis of the bound GTP, the protein undergoes a transition from its GTP-bound form ("reactant", in yellow) to its GDP-bound form ("product", in red). The predominantly mobile regions are the switch I and switch II elements. The gray region is very similar in the two states, and thus can be held fixed during the computation of the path. | Methods to identify MEPs rely on the local optimization of an initially guess for the path. With most initial guesses, these optimization techniques tend to get stuck in local solutions, producing paths with unrealistically high energy barriers. Such paths typically include unphysical transition-states, like the interpenetration of bonds (here of an Arg and a Thr side-chain, top panel), or the passage of a water molecule through an aromatic ring (bottom panel). |
 |

|
Solution:
To obtain an initial guess that can be optimized to a MEP with low
barriers, we used a combined interpolation technique,
interpolating
the coordinates of backbone atoms in Cartesian and the coordinates of
side-chain
atoms in internal coordinates. In addition, the side-chain bonds
are
shrunken in the initial path, resulting in miniature side-chains
which
are unlikely to produce clashes or interpenetrations in the initial
path.
These unnaturally short bonds expand to their normal size in the course
of CPR's optimization procedures.
Results:
After the first CPR refinement, the MEP involves no structural
deformations,
but its rate-limiting energy barrier is still high (~100 kcal/mol).
To reduce this, CPR is run again between local minima adjacent to high
barriers. The new path sections thus produced replaced the old ones.
Following
this strategy, a MEP with a rate-limiting barrier of less than 40
kcal/mol
is found. Repeating this procedure yields a path with a rate-limiting
barrier of only 24 kcal/mol. Enthalpic barriers in the order of
20-40
kcal/mole can be compensated by entropic effects, thus it is possible
that
a realistic pathway has been identified. The final path has more
than 100 transition-states (saddle-points), due to the large
number
of local rearrangements that are necessary for side-chain
re-orientations
and for backbone re-folding, each of which involves at least one
saddle-point.
Molecular movies:
Showing the motion of the backbone, as it unfolds and then
re-folds
the two Switch regions.
The reactant (green) and product
(red) structures are shown throughout the movie to get a feeling of the
structural progress (yellow). One
can
distinguish phases of the transition: A) Unfolding of the Helix
in
Switch II, B) conformational change of Switch I, C) conformational
change
of Switch II. The Mg++ is shown as green sphere, the GDP as
balls&sticks.
Download the movie , 1.7Mb
Showing the motion of the side-chains, which one after the
other
undergo their own re-orientation.
Side-chains are colored according to their instantaneous energy:
brighter colors correspond to higher energies (Dark
blue: energy <= 0. White: energy
>=
30).
The energy assigned to each residue at each frame is computed by its
self-energy plus half the interaction energy with all other residues
(i.e.
these energies add up to the total potential energy). The
difference
between this energy in a given frame and in the end-states gives the
instantaneous
energy.
Download the movie , 3.5Mb.
Same movie, but very fast,
2Mb.