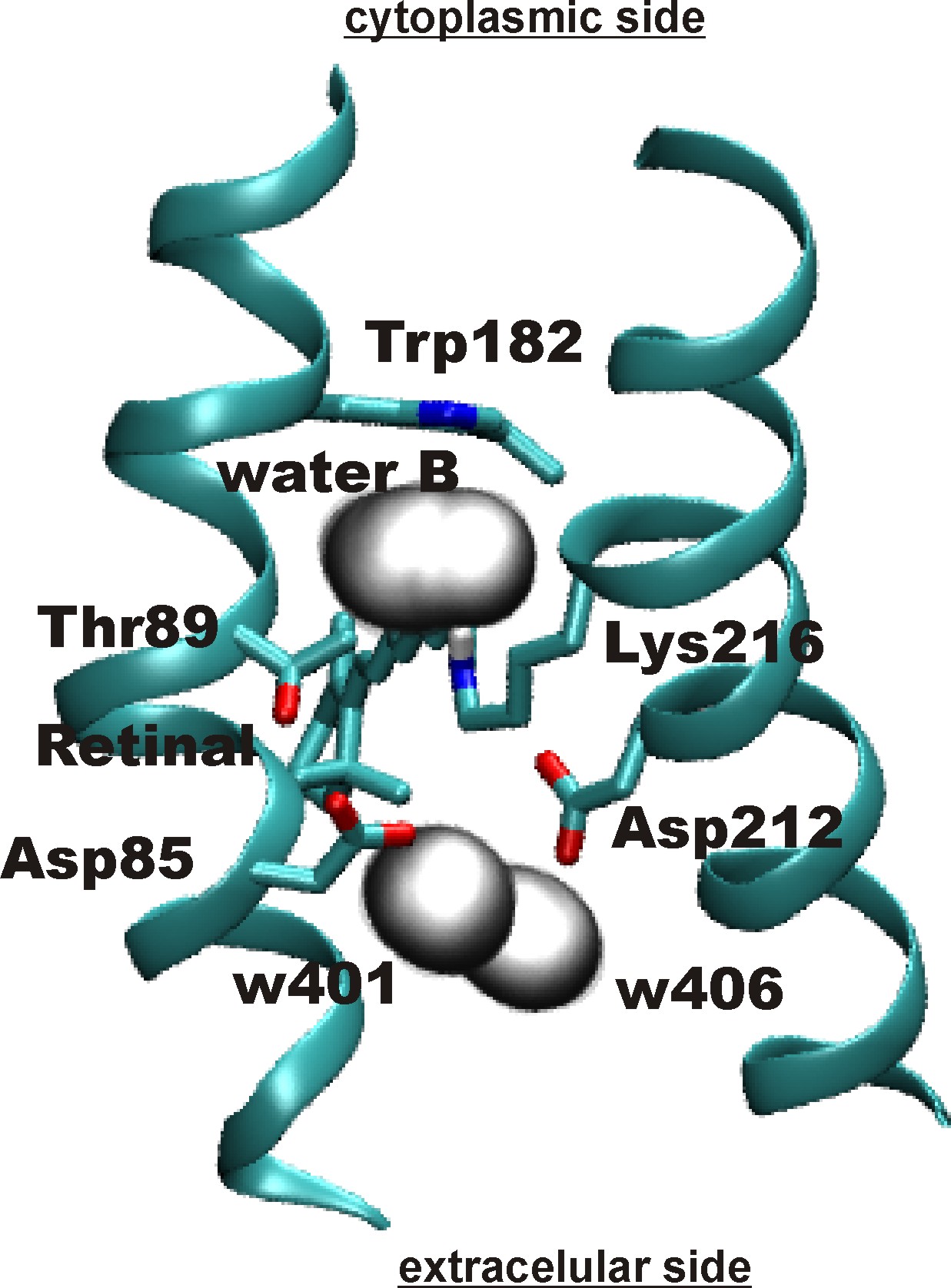
Key role of water: Primary proton transfer
via water-B
in bacteriorhodopsin.
A.N. Bondar, J. Baudry, S. Suhai, S. Fischer, J.C. Smith
J. Phys. Chem. B 112, p. 14729-14741 (2008)
Abstract
The primary proton-transfer step in bacteriorhodopsin is from the
protonated Schiff base (PSB) of retinal to Asp85. One question
concerning the mechanism of this proton transfer step is how the
presence of a water molecule located on the cytoplasmic side of retinal
(called here water B) and making a hydrogen-bond to the PSB would
influence the primary proton transfer.
This is addressed by performing
quantum mechanical/molecular mechanical (QM/MM) reaction path
calculations with the Conjugate Peak
Refinement (CPR) method. These show that the proton transfer
is energetically feasible in the presence of water B: a
low-energy conformation exists in which the positively charged PSB and
the negatively-charged Asp85 are bridged by water B. From this
conformer, concerted proton transfer occurs via water B with a
rate-limiting energy barrier of ~10 kcal/mol.
Molecular dynamics simulations demonstrate that, when water B is
absent, the intrinsic flexibility of the retinal chain enables the
transient formation of a hydrogen bond between the retinal Schiff base
and Asp85, thus making direct proton transfer possible. This confirms
previous reaction path
calculations, which had also shown that such a
transfer is energetically allowed. An alternative route in absence of
water molecule B is via a proton
wire through Asp212, on the other side of the retinal.
|
Location
of water B :
|
Below are shown the
energy profiles of two proton transfers via water B.
Insets give the geometries in the reactant,
product, the rate-limiting transition states, and the local minima
discussed in the paper.
The transferring protons are depicted as small spheres. The reaction
coordinate L gives the sum along the entire path of the
changes in all atomic coordinates calculated as a root-mean-squared
difference: At L=0, the Schiff base is protonated and Asp85 is
negatively
charged; at L=1, Asp85 is protonated and the Schiff base is neutral.
The energies are for QM/MM-optimized geometries.
|
Path 1a: Proton
transfer via
water B.
Download the movie
(1.3 Mb)
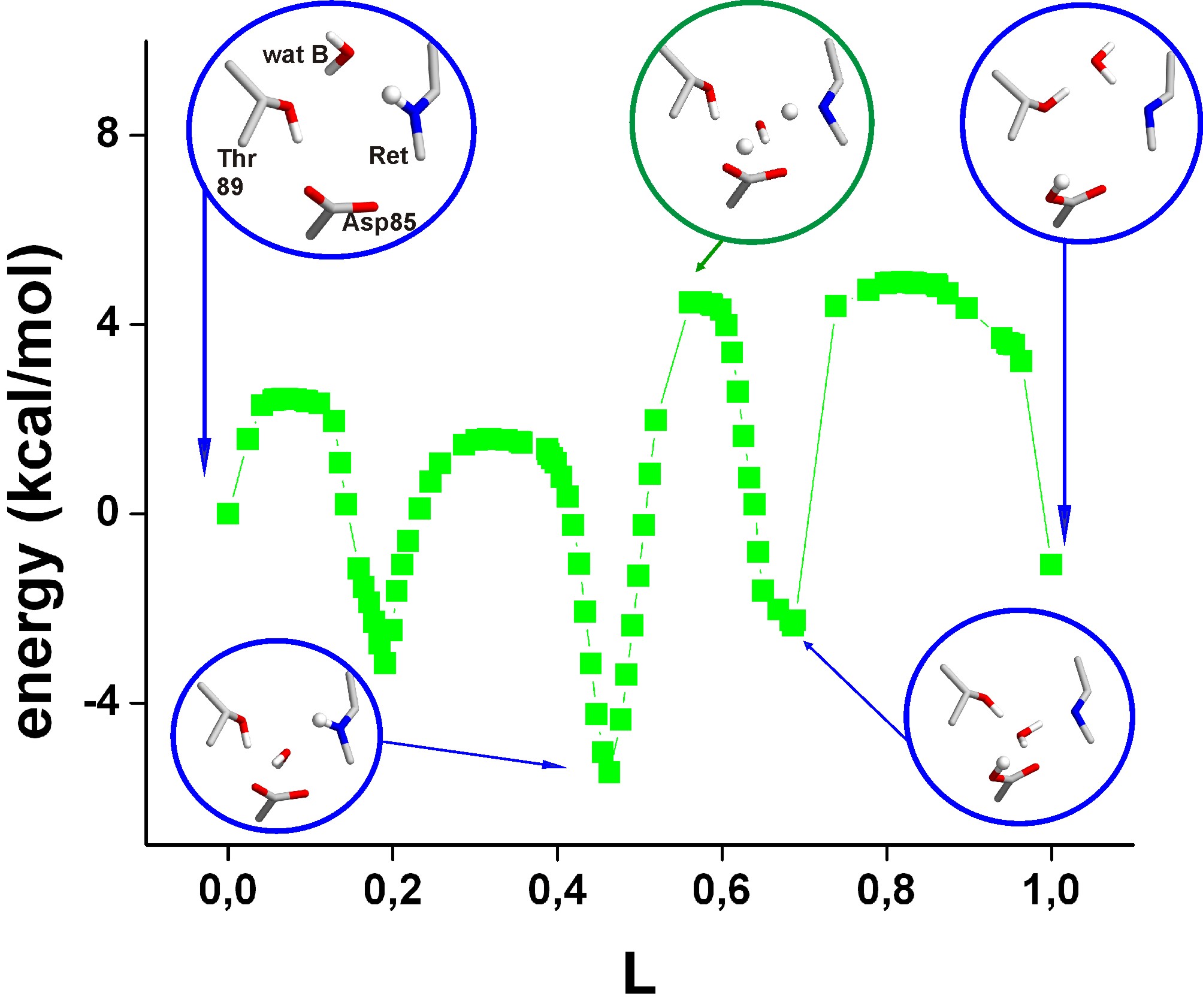
First, water B moves to a
location where it bridges between the Schiff base and Asp85. Then,
concerted proton transfer via water B occurs, crossing a rate-limiting
barrier of 9.7 kcal/mol, which is consistent with the 10 microseconds
experimental timescale of the primary proton transfer step (L to M).
Finally, water B remains hydrogen-bonded to the deprotonated Schiff
base.
Path 2a: Proton transfer via water B and
Thr89.
Download the movie
(1.5 Mb)
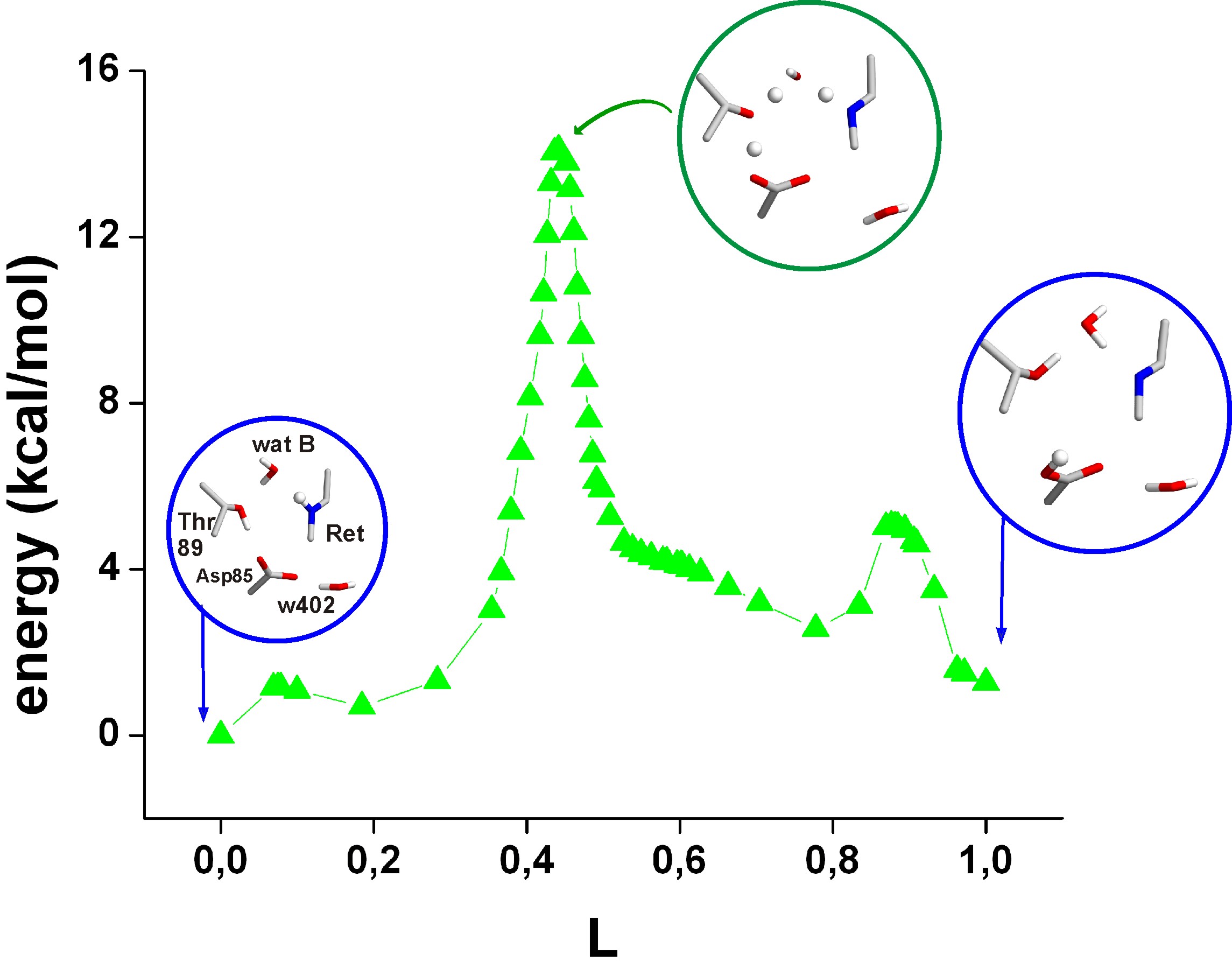
In the starting state, a hydrogen-bonded network already extends
from
the Schiff base to Asp85 via water molecule B and Thr89. Therefore, the
proton transfer requires only minute structural rearrangements,
crossing a rate-limiting barrier of 13.8 kcal/mol.
Go to Home of S. Fischer