Biochemistry
45, p.5830-5847,
2006. (full
PDF)
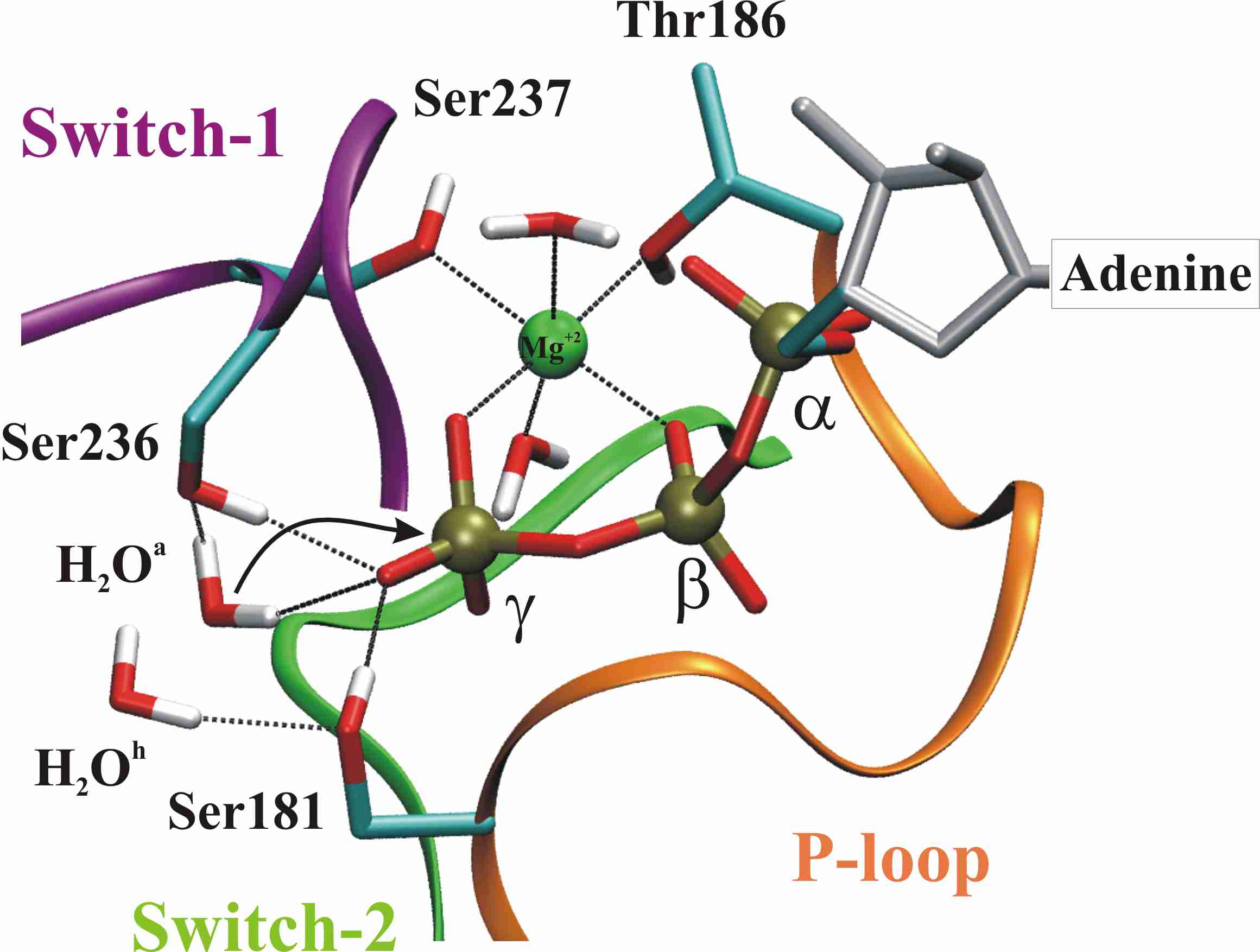
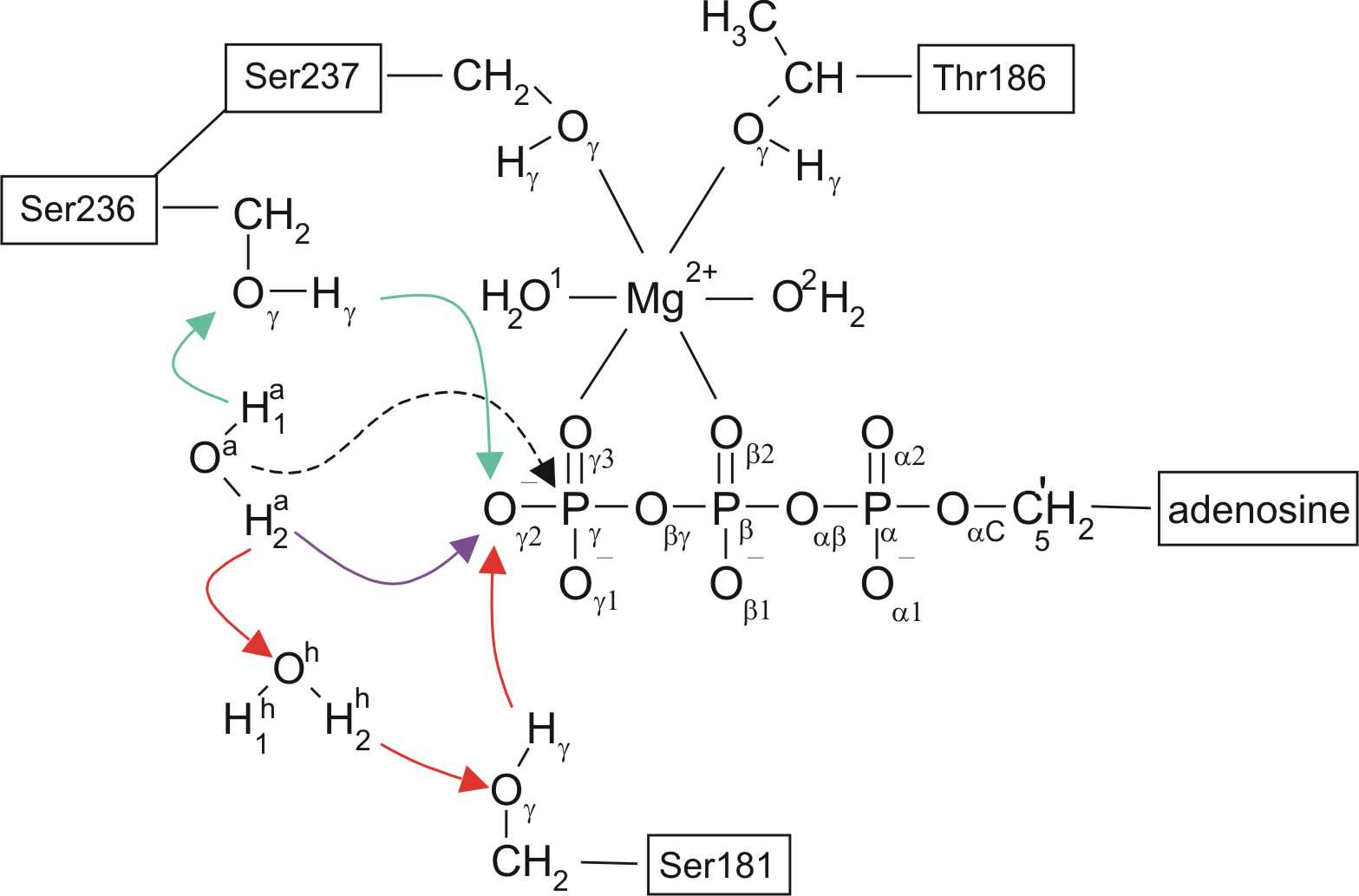
| The ATP-binding pocket in myosin is formed by three loops: the phosphate-binding loop (P-loop), the Switch-1 loop, and the Switch-2 loop . Only the closed Switch-1 / closed Switch-2 (C/C) conformation (shown here) is catalytically active. The arrow shows the attack of the water onto the gamma-phosphate. The connection between Ser237 and the Mg(2+) (green) coordination sphere keeps Switch-1 closed in presence of ATP. The paths show that immediately after hydrolysis, this connection is broken, preparing for the subsequent opening of Switch-1. | Possible reaction pathways for ATP hydrolysis. Three different mechanisms of water activation are shown: the purple arrow corresponds to the direct path, green arrows to the Ser236 path and red arrows to the Ser181 path. The three water activation pathways are found to be equally likely. The dashed arrow shows the attack of the 'activated water' (a) on the gamma-phosphate of ATP. All the atoms treated quantum-mechanically are shown here explicitly. |
 |
 |
"Direct path" :
Activation of the attacking water occurs by direct
proton transfer of its proton to the gamma-phosphate of ATP.
At the transition state, the gamma-phospahate has trigonal bipyramidal
geometry.
In the product state, the Mg(2+)
moves between the beta and gamma phosphates, and away from Ser237,
thereby breaking
its coordination bond to the Switch-1 loop. This weakens
one
of the strong interactions that maintain Switch-1 closed over the
nucleotide.
Download the movie (1.2 Mb)
"Ser236 path" :
Ser236 transfers its proton onto the gamma-phosphate, and the
'attacking
water' (a) reorients into a position optimal for the transfer of its
proton
to Ser236. The transition and product states are similar to the
ones
descibed for the "direct" path.
Download the movie (1.3 Mb)
"Ser181 path" :
Ser181 transfers its proton to the gamma-phosphate, the 'helper water'
(h) transfers a proton onto the sidechain of Ser181, and the 'attacking
water' (a) transfers a proton onto the helper water. The
transition
and product states are similar to the ones descibed for the "direct"
path.
Download the movie (2.1 Mb)