Structural mechanism of the recovery stroke
in the
Myosin molecular motor.
Stefan Fischer*, Bjoern Windshuegel, Daniel Horak,
Kenneth C. Holmes and Jeremy C. Smith.
Proceedings of the National
Academy
of Sciences USA, vol. 102,
6873-6878 (2005)
Abstract
During muscle contraction, after the force-generating power-stroke and
ATP-induced
unbinding from actin, myosin swings back its lever-arm by ~60
degrees. During this "recovery stroke" it must
accomplish
a crucial function, which is to couple its ATPase activation to the
large
rotation of the "converter" domain that carries the lever arm.
Here,
the atomic details of this transition
are
determined by computing a minimum-energy path for the recovery-stroke
transition,
using the
Conjugate Peak Refinement (CPR) method.
The path reveals how a series of structural changes along the
"relay"
helix amplify small changes near the ATP binding site into the
large motion
of the lever arm.
This has lead to a comprehensive picture of this essential chemo-mechanical coupling.
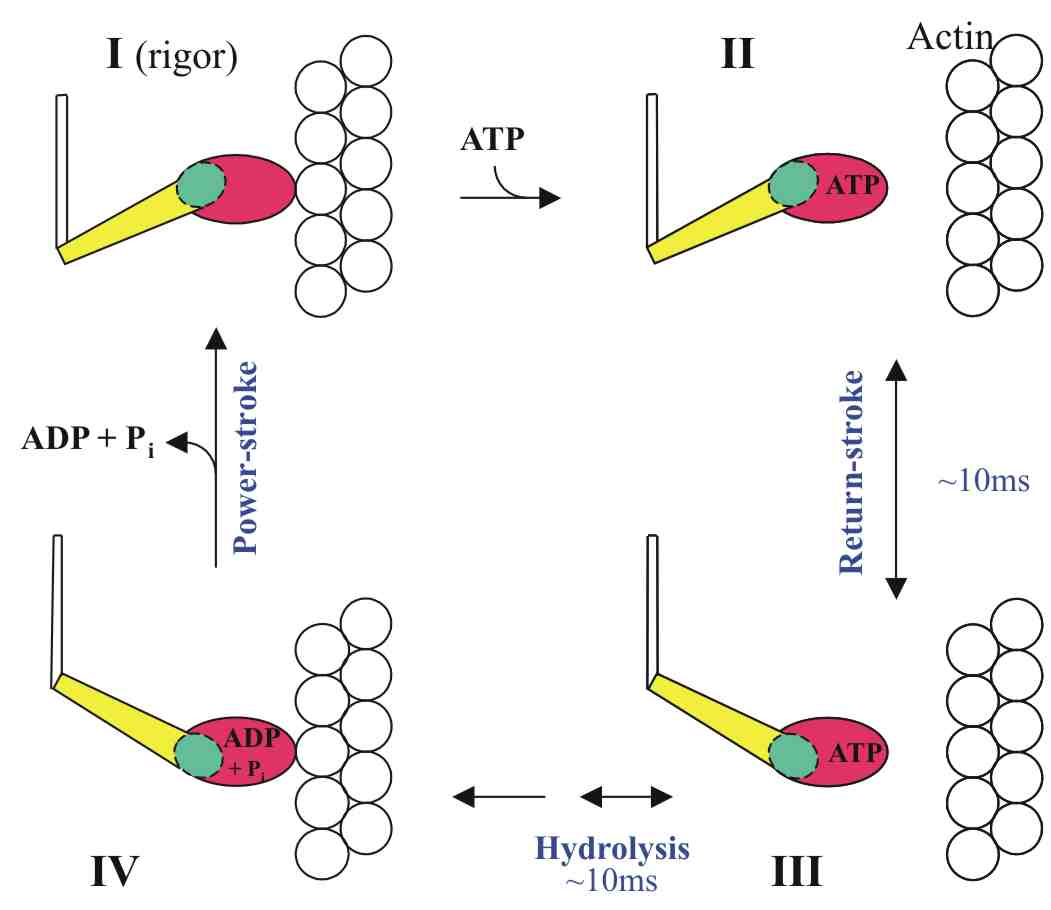
The Lymn-Taylor contraction cycle.
The recovery-stroke is between states II and III: the
lever arm (in yellow) attached to the converter domain (in green)
swings
back to prepare for the next power-stroke. |
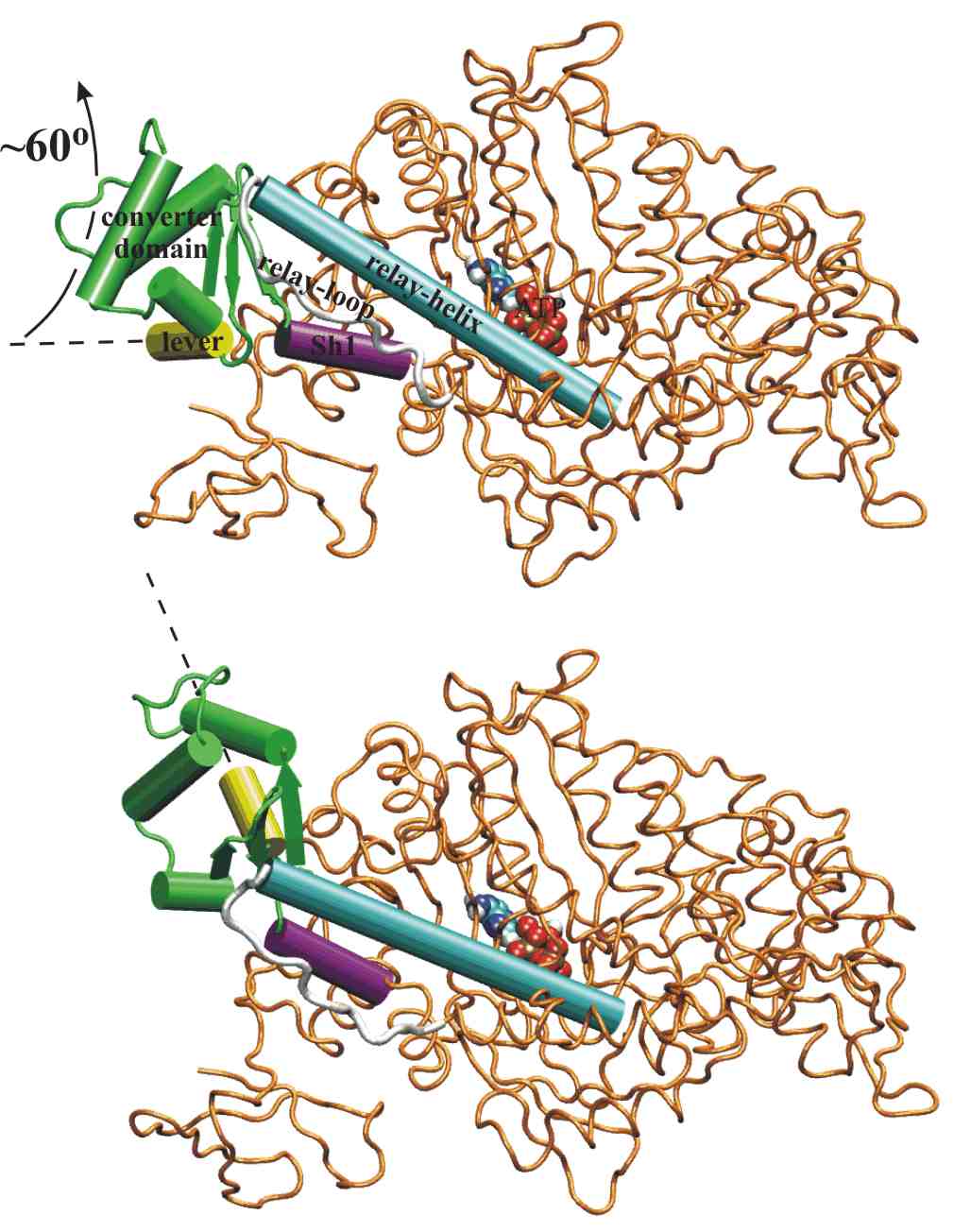
The two end-state structures
of the recovery-stroke.
The converter domain (in green) rotates by ~60 degrees to swing the
lever-arm (in yellow). It is 40Angstroem away from the ATP (in
van
der Waals spheres). The converter-domain orientation must
be coupled to the conformation of the relay helix (in cyan)
near
the ATP in such a way that ATPase
activity is switched on only when
the Lymn-Taylor cycle is in state III. |
|
 |
Movie 1. Overall view (Fig1 B&C of
paper):
Overall view showing the recovery stroke of Myosin. The converter
domain (green) and the lever-arm (yellow) are seen to rotate ~65
degrees
relative to the main body of myosin (orange). After the end of
this
movie, the actin fibril (not shown) comes from the right side and
re-binds
to the main body for the power stroke. The movie frames are made
from coordinate sets taken from the minimum energy path (MEP) computed
by Conjugate Peak Refinement (CPR), as described in the paper.
Here,
only the protein backbone (colored as in Figure 1B&C) and the bound
ATP (in van der Waals) are shown. File size 1Mb.
Download the movie
(Low-res. version, 1Mb, as in PNAS suppl. materials)
Download the movie
(High-res. version, 2.8Mb)
Movie 2.
Same as movie 1, but showing all the atoms used in the calculation.
Download the movie
(Large file, 12Mb, as in PNAS suppl. materials)
Movie 3. Hinging of the converter domain
(see
Fig.2-left of paper).
Motions described in Figure 2. As movie 1, but different view
(down the SH1-helix, in purple) showing the converter domain (green)
and
the lever-arm (yellow) rotating ~65 degrees relative to the main body
of
myosin (orange), around an axis close to the axis of the
SH1-helix.
Shows the hinging point of the converter domain on the SH1-helix, and
the
linkage between the converter domain and the C-terminus of the relay
helix
(in cyan).
Download the movie
(Low-res.
version, 1.5Mb, as in PNAS suppl. materials)
Download the movie
(High-res. version, 5Mb)
Movie 4. Seesaw and local unwinding of the
relay helix (see
Fig.2-right
of paper).
Motions described in Figure 2 (same view and coloring as in movie
1). Shows how the closing of the Switch2 loop (orange) over the
bound ATP (van der Waals) is coupled to a large translation of the
C-terminus
of the relay helix (cyan), which accompanies the rotation of the
converter domain (of which a small
piece
is shown in green). To accomodate this rotation, the C-terminal
third of the relay helix undergoes an unwinding by 1/8th turn, breaking
the helical H-bond at residue 486. As a result of the unwinding, the
aromatic ring of Phe487 (orange) reorients and must thread between the
relay helix and the relay loop (in white).
Download the movie
(Low-res. version, 0.7Mb, as in PNAS suppl. materials)
Download the movie
(High-res. version, 8.5Mb)
Validation
The seesaw motion of the relay helix that is seen during the
present CPR pathway is also present (with a smaller amplitude) in the
spontaneous fluctuations of that helix during equilibrium Molecular
Dynamics (MD) simulations of the pre-recovery state (State II). This
was shown by performing Principal
Component Analysis of the relay helix motions, thus providing
strong evidence in favor of the proposed mechanics of the
recovery-stroke.
Go to Home of S. Fischer

