The principal motions involved in the
coupling
mechanism of the recovery stroke of the
Myosin motor.
S. Mesentean, S. Koppole, J.C. Smith, S. Fischer*
J. Mol. Biol., vol. 367, p. 591-602 (2007)
Abstract
Muscle contraction is driven by a cycle of conformational changes in
the myosin II head. After myosin binds ATP and releases from the actin
fibril, myosin prepares for the next power stroke by rotating back the
converter domain that carries the lever arm by ~60 degrees. This recovery stroke
is coupled to the activation of myosin's ATPase by a mechanism that is
essential for an efficient motor cycle. The mechanics of this coupling
have been proposed to occur via two distinct and successive motions of
the two helices that hold the
converter domain: in a first phase a see-saw motion of the relay helix,
followed by a piston/seesaw motion of
the SH1 helix in a second phase. To test this model, we have
determined the principal motions of these structural elements during equilibrium molecular dynamics
simulations of the crystallographic end states of the recovery stroke
by using Principal Component Analysis.
This reveals that the only principal motions of these two helices that
make a large amplitude contribution towards the conformational change
of the recovery stroke are indeed the predicted seesaw and piston
motions.
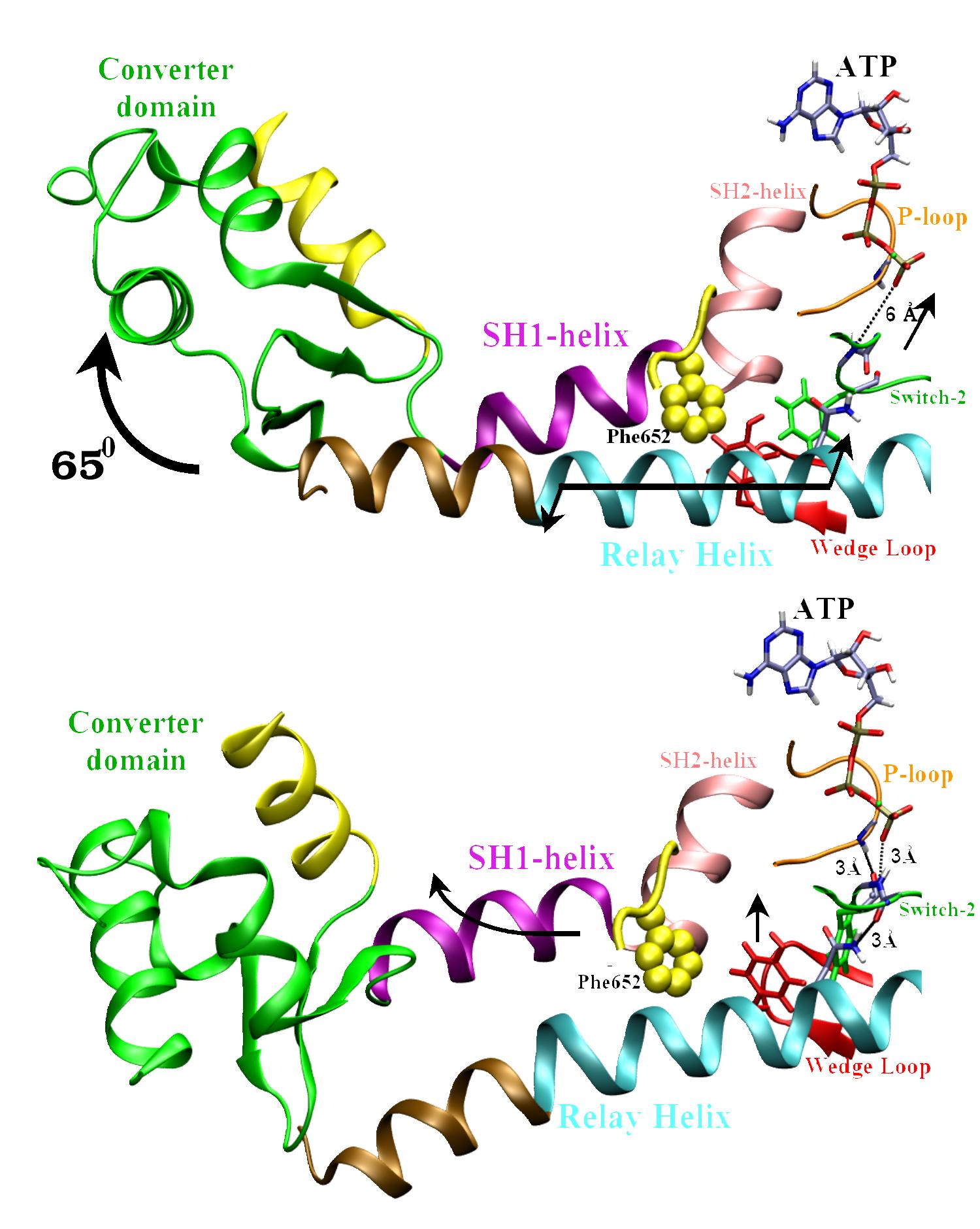
The two end-state conformations of the
recovery stroke.
The converter domain rotates by ~65 degrees to swing the
lever arm (in yellow). This rotation is coupled to the closing of the Switch-2 loop over
the 40 Angstrom distant ATP. The closing of Switch-2 forms two key H-bonds: 1) Between Gly457
and ATP, and 2) between Phe458 and the Phosphate loop (a.k.a. P-loop),
which turn on the ATPase
activity of Myosin. |
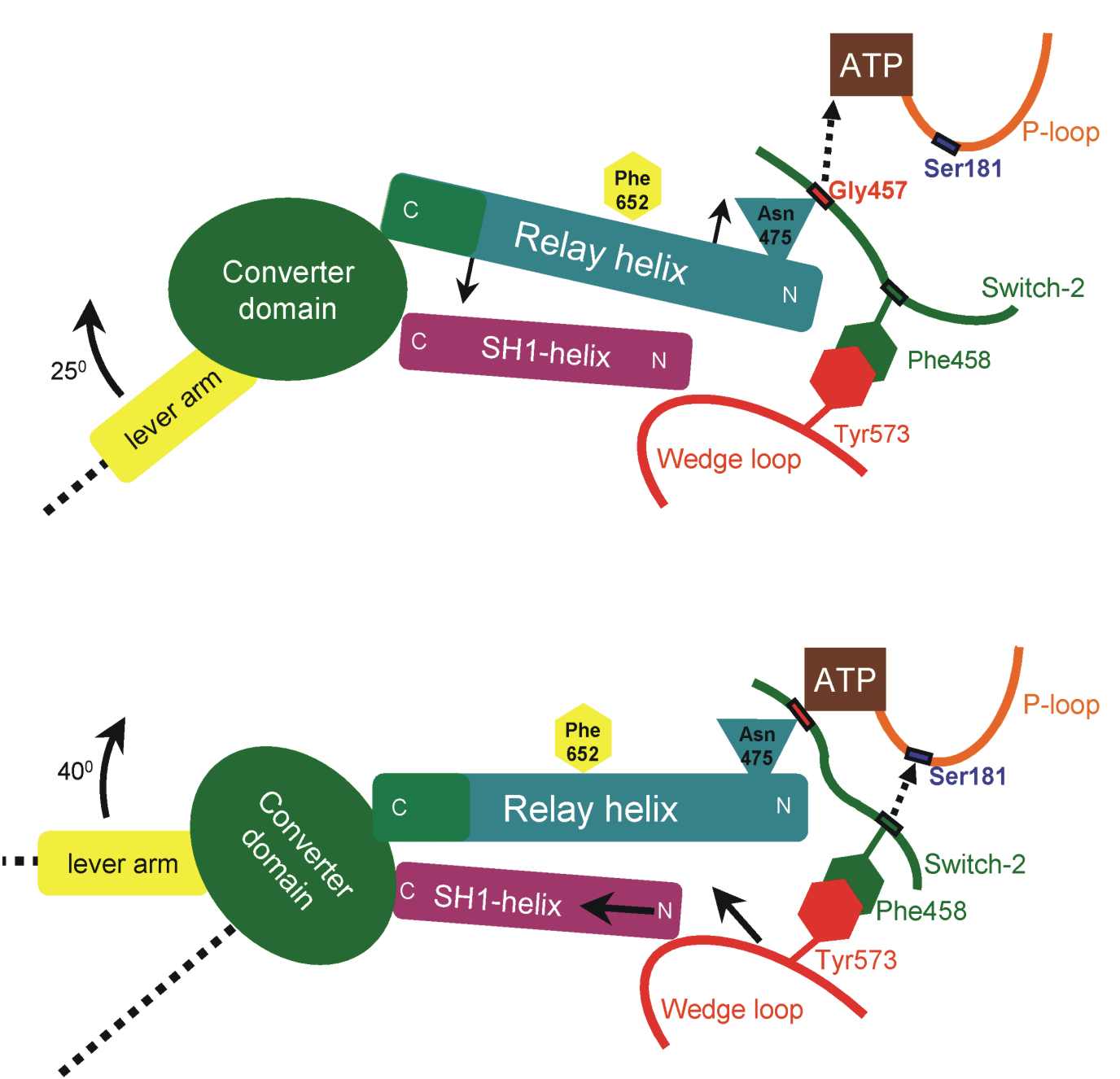
The coupling
model has two phases.
Phase-I: Formation
of the H-bond between Gly457 and ATP pulls the relay helix and causes
its seesaw pivoting (see
arrows). The converter domain attached at the C-terminal-end
of the relay helix reacts with a rotation of 25 degrees.
Phase-II: The
H-bond between Phe458 and the P-loop pulls along the "Wedge-loop"
(residues 572-574), which makes tight hydrophobic interactions with
Phe458 of the
Switch-2 loop. The Wedge loop pushes the SH1-helix, which responds with
a piston/seesaw motion. The
converter domain, which is attached to the SH1-helix,
reacts with a 40 degree
rotation.
|
|
 |
The coupling model is checked by analyzing the motions of the
implicated elements (converter domain, relay and SH1 helices, wedge
loop) during equilibrium molecular
dynamics (MD) of the protein in the two crystallographic
endstates of the recovery stroke.
The principal motions, i.e., the deformations of largest amplitude that
occur during the MD, are identified by Principal
Component Analysis (PCA). The principal motions that contribute
most to the recovery-stroke transition are shown in the following
movies. The color-coding in all the movies is the same as in the
figures shown above. Each movie shows one complete cycle of
oscillatory motion and is best viewed by setting the Movieplayer on "Auto-replay".
Movie 1. Converter
domain rotation:
Principal motion of the converter domain in the MD of the post-recovery
conformation (Principal Component #1, PC1). It consists in a
partial rotation of the converter domain (by ~8 degrees) around an axis
parallel to the SH1 helix. This rotation is accompanied by a
translation of the C-terminus of the relay helix. The amplitude shown
in this movie is the same as the amplitude
observed in the MD at room temperature.
The motions of PC1 correspond to the expected rotation of the converter
domain and swinging of the lever-arm.
Download the movie
(1Mb)
Movie 2. Seesaw motion of the relay helix:
Principal motion of the relay helix in the MD of the pre-recovery
conformation (PC#2). The atoms at one end of the helix swing in
the direction opposite to the direction of the atoms at the other end,
while the stationary point of the relay helix is located in the middle
of the helix, where Phe652 (in yellow) is the pivoting point of the
seesaw.
Download the movie
(1Mb)
Movie 3. Correlated wedge/piston motion of
the wedge-loop/SH1-helix:
Principal motion of the SH1 and SH2 helices plus the Wedge-loop in the
MD of the post-recovery conformation (PC#3). The atoms of the
wedge-loop move towards the corner between the
SH1 and SH2 helices, whose atoms move together towards the converter
domain, undergoing a piston-like motion. This is the correlated
motion that is predicted by the
coupling model near the end of the recovery stroke.
Download the movie
(1Mb)
Conclusions
The present results provide strong evidence in favor of the
proposed mechanics of the recovery stroke and the related coupling
model (see also the paper).
Go to Home of S. Fischer

