The structural coupling between ATPase
activation and recovery-stroke in the Myosin II motor.
S. Koppole, J.C. Smith, S. Fischer*
Structure, vol. 15, p.825-837 (2007)
Abstract
Muscle contraction is driven by a cycle of conformational changes in
the myosin II head. Before the Myosin motor head can perform the next
power-stroke, it undergoes a large conformational transition in which
the converter domain (which bears the lever arm) rotates by ~65
degrees. Simultaneously to this "recovery stroke", myosin
activates its ATPase function
by closing the Switch-2 loop
over the bound ATP. This coupling between the motion of the converter
domain and the motion of the 40Å distant Switch-2 loop is
essential for the
motor cycle since hydrolysis of the ATP is required for subsequent
actin rebinding. The coupling
mechanism is determined by finding a series of optimized
intermediates between the crystallographic end-structures of the
recovery stroke, yielding movies of the transition at atomic detail.
This reveals a two-phase
mechanism, in which the successive formation of two hydrogen bonds by the Switch-2 loop
is correlated with the successive see-saw
motions of the two helices that hold the converter domain: the relay helix and the SH1-helix. The converter domain
responds to the relay see-saw by rotating 25 degrees, then to the SH1
see-saw by rotating a further 40 degrees.
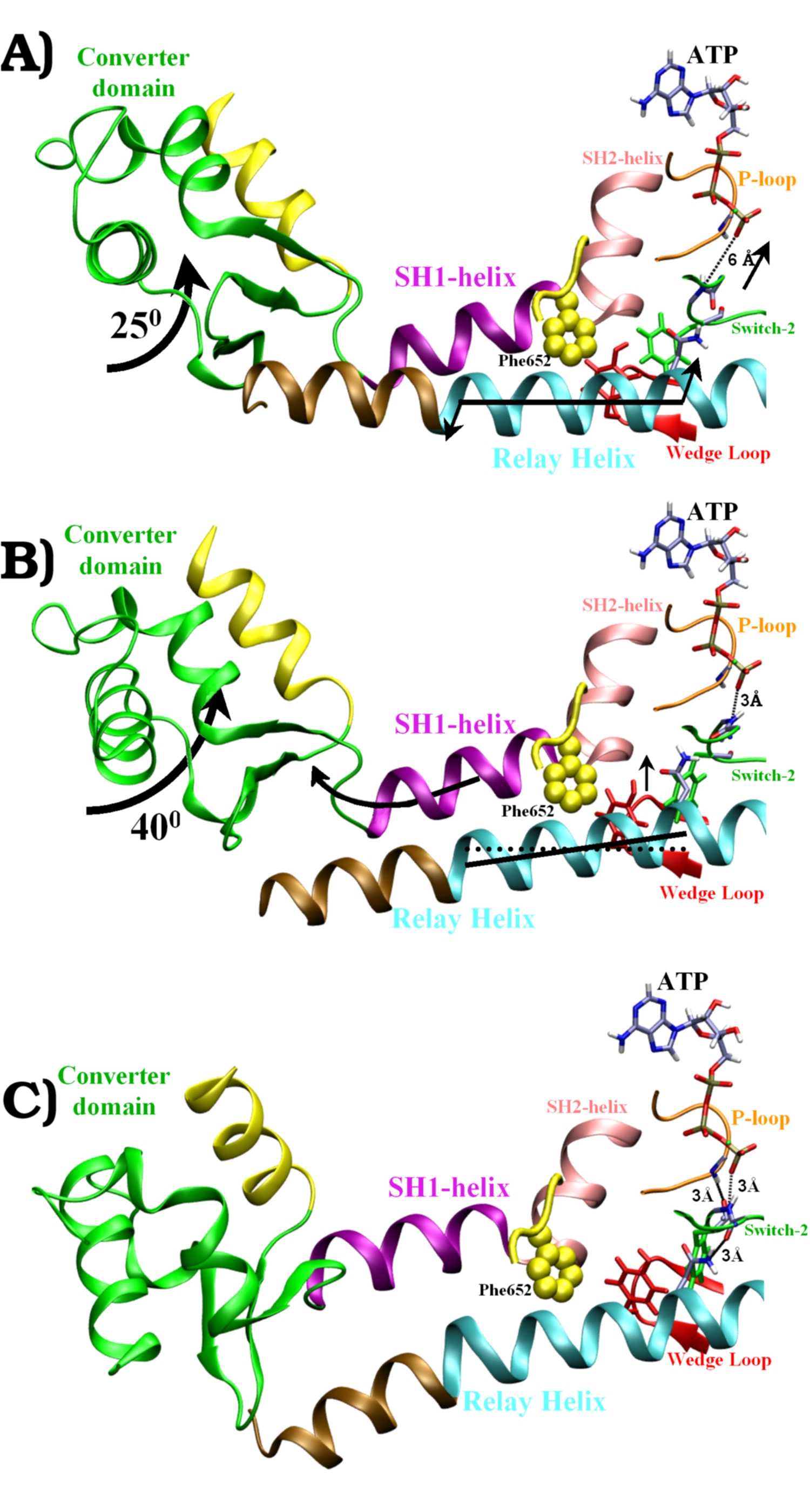
Structures
along the recovery-stroke pathway:
A) Pre-recovery state. The
Switch-2 loop is fully open, the relay helix is straight. Arrows show
the motions of Phase I.
B) Half-way along the transition
pathway (converter domain rotated 25 degrees). The Switch-2 loop is
half-closed (H-bond between Gly457 and ATP). The tilt of the relay
helix relative to its orientation in (A) is indicated by the straight
dotted line. Arrows show the motions of Phase II.
C) Post-recovery state
(converted domain rotated 65 degrees). The Switch-2 loop is fully
closed. The Wedge-loop has moved up against the SH1-helix. The relay
helix is "kinked" (between the brown and the cyan coloring).
The closing of Switch-2 forms two key
H-bonds: First between Gly457 and ATP (A->B), and then
between Phe458 and the P-loop (B->C). This closing turns on the ATPase
activity of Myosin. |
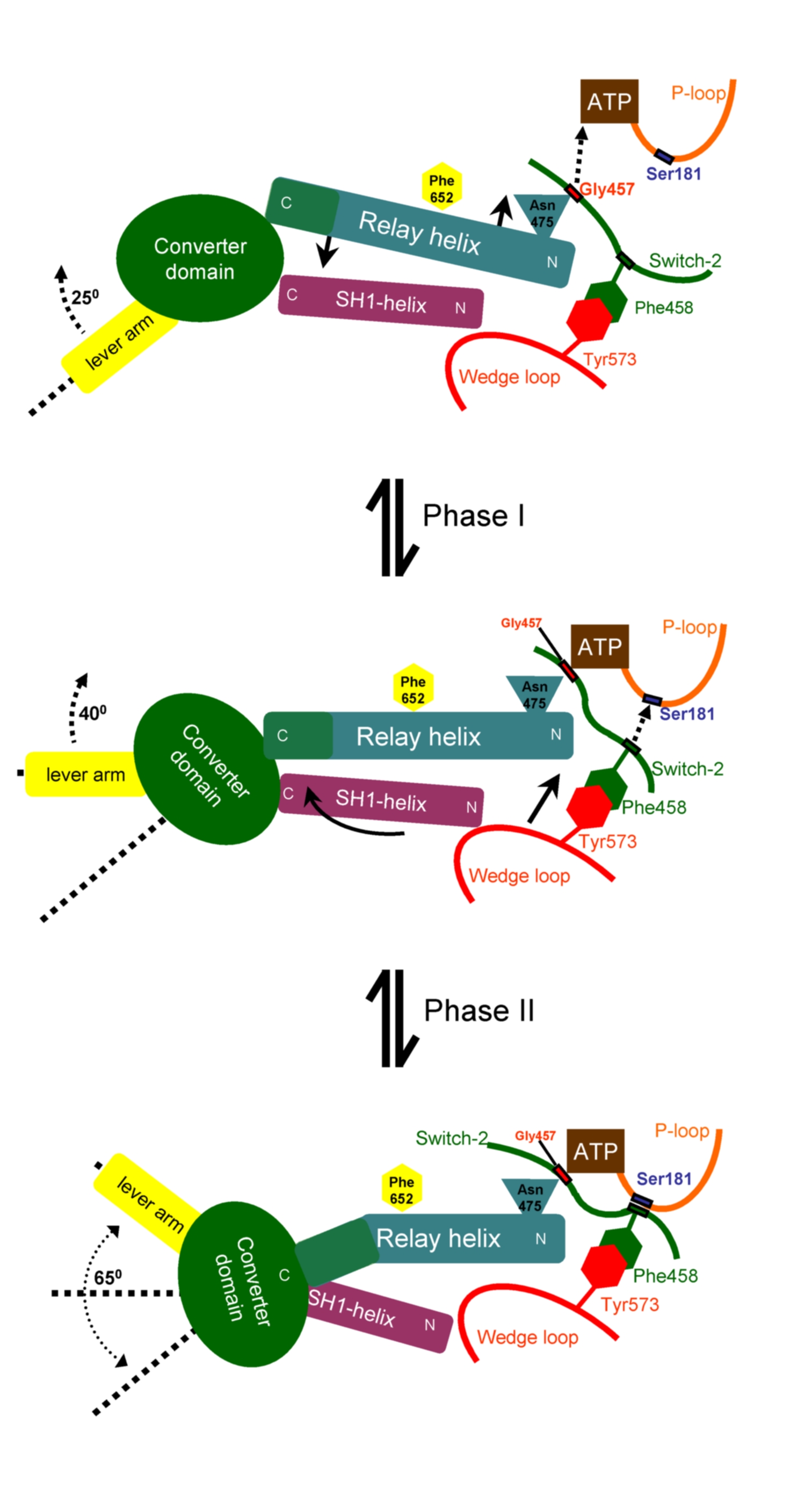
The coupling model
has two phases:
Phase-I. Formation
of the H-bond between Gly457 and ATP (dotted arrow) pulls the relay
helix and causes
its seesaw pivoting (small
full
arrows). The converter domain attached at the C-terminal-end
of the relay helix reacts with a rotation of 25 degrees.
Phase-II. The
H-bond between Phe458 and the P-loop (dotted arrow) pulls along the
"Wedge-loop"
(straight full arrow), which makes tight hydrophobic interactions with
Phe458 of the
Switch-2 loop. The Wedge loop pushes the SH1-helix, which responds with
a piston/seesaw motion (curved
full arrow). The
converter domain, which is attached to the SH1-helix,
reacts with a 40 degree
rotation.
|
|
 |
Movies
"lambda" measures the
progress
along the transition pathway from pre-recovery (0%) to post-recovery
(100%).
The color-coding in all the movies is the same as in the
figures shown above: Converter domain in green, lever arm in
yellow, SH1-helix in purple, SH2-helix in pink, Relay helix in cyan
(C-terminal third in brown), Switch-2 loop in green, P-loop in orange,
Wedge loop in red, Phe652 in yellow.
Movie 1. Pathway of
the whole recovery stroke
(lambda = 0% - 100%).
Shows all structural elements involved.
Download the movie
(1.8Mb)
Movie 2. Two-step closing of the Switch-2 loop
(lambda = 0% - 100%).
Shows first the formation of the hydrogen bond between Gly457 and the
gamma-phosphate, then the formation of the hydrogen bond between Phe458
and Ser181.
Download the movie
(1.8Mb)
Movie 3. Seesaw of the relay helix in Phase
I.
Shows the motion forward and back (i.e., lambda = 0% - 20% -
0%, to be viewed in auto-replay
mode).
Download the movie
(0.6Mb)
Movie 4. Phase II of the
transition (lambda = 20% - 100%).
Shows the formation of the Phe458/Ser181 hydrogen bond, the
wedging of the Wedge loop against the SH1/SH2 corner and the
seesaw/piston motion of the SH1-helix.
Download the movie
(1Mb)
Movie 5. Seesaw of the SH1 helix in Phase
II.
Shows the motion forward and back (i.e., lambda = 65% - 100%
- 65%, view in autoreplay
mode).
Download the movie
(0.8Mb)
Conclusions
Principal component analysis
during equilibrium molecular dynamics simulations revealed that the
relevant principal motion of the relay helix during MD of the
pre-recovery state is indeed the seesaw pivoting predicted in Phase I,
while the relevant principal motion of the SH1-helix found during MD of
the post-recovery state is the piston/seesaw motion described here for
Phase II of the coupling mechanism.
Go to Home of S. Fischer

